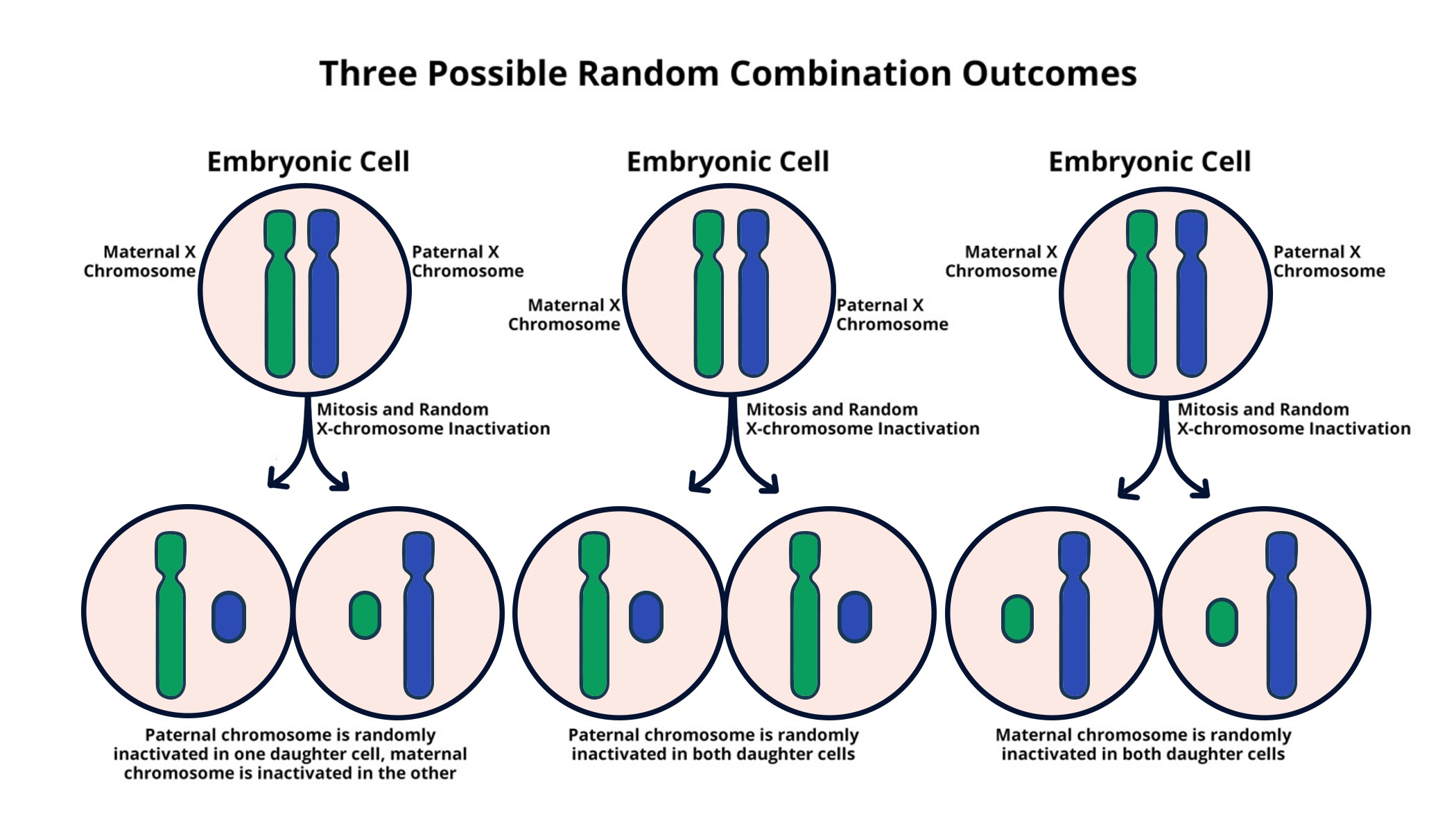
X chromosome inactivation (XCI) is a remarkable biological process that plays a crucial role in the genetic landscape of females. Unlike males, who possess a single X chromosome, females contain two, necessitating the inactivation of one to maintain genetic balance. Understanding how XCI operates has far-reaching implications, particularly for individuals affected by genetic diseases such as Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Jeannie Lee’s innovative research at Harvard Medical School sheds light on the complexities of this process, revealing potential pathways to develop chromosome therapy for these disorders. As scientists delve deeper into the mechanisms of XCI, the promise of new treatments for debilitating genetic conditions draws closer.
The phenomenon of X chromosome inactivation, also known as Lyonization, presents a unique challenge in the realm of genetics, particularly due to its association with various genetic disorders. In females, the presence of two X chromosomes leads to a necessity for inactivation to prevent gene dosage imbalances, a process that becomes critical in understanding conditions like Fragile X Syndrome and Rett Syndrome. Recent breakthroughs in chromosome therapy, spearheaded by researchers such as Jeannie Lee at Harvard, illuminate the steps involved in silencing one of these X chromosomes. As we explore the cellular mechanisms and the role of innovative therapies in addressing these genetic diseases, the potential to alleviate the burden of X-linked conditions becomes increasingly tangible. The ongoing studies not only promise to revolutionize treatment strategies but also enhance our comprehension of XCI and its implications in human health.
Understanding X Chromosome Inactivation: A Breakthrough in Genetic Research
X chromosome inactivation, a crucial biological process, refers to the silencing of one of the two X chromosomes present in female cells. This phenomenon ensures that the gene dosage remains balanced between males and females, despite the females having two X chromosomes. Jeannie Lee’s research has significantly advanced our understanding of how this complex mechanism operates. The inactivation process involves intricate interactions between genes and a gelatinous coating—referred to as ‘chromosomal Jell-O’—which facilitates the silencing of one X chromosome. These interactions highlight how critical this process is for maintaining cellular function and genomic stability in women.
Inactivation begins when genes on the X chromosome produce an RNA molecule named Xist, which plays a pivotal role in modifying the properties of the surrounding chromosomal matrix. Studies conducted by Lee’s team reveal that this gelatinous substance needs to be flexible enough to allow Xist and various other molecules to infiltrate and effectively silence the chromosome. This operational mechanism not only serves as a foundational biological insight but may also lead to innovative therapeutic approaches for genetic disorders tied to the X chromosome. The ongoing exploration in these areas holds promise for advancing treatments for conditions such as Fragile X Syndrome and Rett Syndrome.
The Role of Chromosome Therapy in Treating Fragile X and Rett Syndromes
As research into chromosome therapy evolves, its potential applications in treating genetic disorders like Fragile X Syndrome and Rett Syndrome have become more apparent. Fragile X Syndrome, characterized by intellectual disability, stems from mutations within the FMR1 gene on the X chromosome. Similarly, Rett Syndrome, which predominantly affects females, is linked to mutations in the MECP2 gene also found on the X chromosome. By understanding the mechanisms of X chromosome inactivation, Lee’s research opens doors to therapies that could reactivate the healthy gene while silencing the mutated one. This could potentially reverse symptoms associated with these debilitating conditions.
Chromosome therapy aims to harness the power of Xist and its ability to manipulate the chromosomal environment to unsilence the inactivated X chromosome bearing the healthy gene. This transformational approach offers a dual benefit—it aims to restore normal function to mutated genes while minimizing the risk of impacting other, healthy genes. Lee’s team is actively optimizing these therapeutic techniques, laying the groundwork for clinical trials that could provide much-needed relief for those plagued by these genetic diseases. As more data supports the efficacy and safety of these gene therapies, the hope for a future cure becomes more tangible.
Exploring Genetic Diseases Linked to the X Chromosome
Genetic diseases associated with the X chromosome, such as Fragile X Syndrome and Rett Syndrome, pose significant challenges for individuals and families. Fragile X, caused by an expanded CGG repeat in the FMR1 gene, accounts for a substantial percentage of hereditary intellectual disabilities. On the other hand, Rett Syndrome, primarily affecting females, leads to severe cognitive and physical impairments due to defects in the MECP2 gene. The understanding of X chromosome inactivation is particularly important in advancing treatment strategies as many of these disorders are linked to dysfunctional gene activity due to mutations that prevent normal gene expression on the X chromosome.
Research efforts spearheaded by scientists like Jeannie Lee are critical in demystifying how the processes surrounding X chromosome inactivation could be harnessed for therapeutic ends. By exploring how Xist regulates chromosomal silencing, researchers aim to identify methods for effectively reactivating healthy genes while leaving harmful mutations silenced. This dual approach holds promise not just for treating specific conditions, but also for potentially uncovering broader applications in genetic medicine aimed at other chromosomal disorders. With ongoing investigations in chromosome therapy, there is genuine hope for transformative outcomes for patients experiencing the impacts of these genetic diseases.
Jeannie Lee’s Pioneering Research at Harvard Medical School
Jeannie Lee’s pioneering work at Harvard Medical School has placed her at the forefront of chromosome biology. Her lab has made significant strides in unraveling the complexities of X chromosome inactivation, a process that has direct implications for a variety of genetic diseases. For decades, researchers have struggled to understand how cells silence one X chromosome in females, involving intricate molecular interactions and dynamics. Lee’s recent findings bring clarity to how the gelatinous substance surrounding chromosomes, likened to ‘Jell-O’, plays a crucial role in facilitating this silencing. Her research could elucidate pathways for therapeutic strategies targeting X-linked disorders.
Additionally, Lee emphasizes the importance of continuing to explore these cellular mechanisms, noting that the journey from understanding fundamental biological processes to developing clinical therapies can take time and rigorous investigation. With funding from the National Institutes of Health over the years, her research trajectory has begun to reveal potential treatments aimed at unsilencing genes affected by mutations. As her team progresses toward clinical trials, their work may hold the key to unlocking effective therapies for conditions currently deemed untreatable, leveraging the innate mechanisms of X chromosome behavior.
The Future of Genetic Treatments: Implications of Chromosomal Research
The future of genetic treatments is intimately tied to ongoing research regarding chromosomal dynamics, particularly in the context of X chromosome inactivation. As scientists like Jeannie Lee delve deeper into the mechanisms of gene silencing and activation, a new realm of possibilities for treating genetic diseases, especially those influenced by X-linked mutations, emerges. The potential to free inactivated X chromosomes signifies hope for individuals with genetic conditions like Fragile X Syndrome and Rett Syndrome, where the healthy gene is locked away due to the inactivation process. This transformative approach could fundamentally alter the therapeutic landscape.
Moreover, the implications extend beyond merely treating symptoms; they suggest a paradigm shift towards potentially curing genetic diseases. By strategically manipulating the inactivation processes, researchers aim to restore functionality to affected genes while keeping the risks of affecting surrounding healthy genes to a minimum. As additional data is gathered through safety studies and early clinical trials, the scientific community anticipates that these methods will usher in a new era of chromosome therapy, characterized by precision and efficacy that could redefine the management of genetic diseases associated with the X chromosome.
Deciphering Chromosomal Jell-O: A New Perspective on Genetic Disorders
The concept of ‘chromosomal Jell-O’ as described by Jeannie Lee emphasizes the importance of biophysical properties in understanding gene expression and regulation. This gelatinous substance not only helps maintain the structural integrity of chromosomes but also influences the accessibility and activity of genes located on these chromosomes, particularly in processes such as X chromosome inactivation. By exploring how this Jell-O interacts with regulatory molecules like Xist, researchers can gain insights into how certain genes are silenced or activated, including those responsible for genetic diseases.”},{
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic diseases?
X chromosome inactivation is a biological process wherein one of the two X chromosomes in female mammals is randomly silenced to balance gene dosage between males and females. This process is crucial for preventing genetic diseases linked to mutations in X-linked genes, such as Fragile X Syndrome and Rett Syndrome. Understanding this mechanism provides insights into potential therapies for these conditions.
How does Xist contribute to X chromosome inactivation?
The Xist gene, located on the X chromosome, produces a non-coding RNA that plays a key role in X chromosome inactivation. It coats the inactive X chromosome, altering the chromosomal environment, which helps in silencing the genes on that X chromosome. This process is critical for ensuring that only one X chromosome is active in female cells, thereby preventing the overexpression of X-linked genes.
What are the implications of Jeannie Lee’s research on X chromosome inactivation for treating Fragile X Syndrome?
Jeannie Lee’s research on the mechanisms of X chromosome inactivation opens pathways for potential therapeutic treatments for Fragile X Syndrome. By unsilencing inactivated X chromosomes, her team aims to restore the functionality of healthy genes, which can alleviate symptoms associated with this genetic disorder.
What role does chromosomal structure play in X chromosome inactivation?
The chromosomal structure, influenced by a gelatinous substance described by Jeannie Lee’s team as ‘chromosomal Jell-O,’ is vital for X chromosome inactivation. This substance allows the X chromosome to be coated by Xist RNA and other proteins, creating an environment that facilitates the silencing of the inactive X chromosome, crucial for managing genetic diseases linked to this chromosome.
Why is X chromosome inactivation a challenge for males with X-linked genetic disorders?
While males typically have only one X chromosome and do not undergo X chromosome inactivation, certain X-linked genetic disorders like Fragile X Syndrome can still affect them. In males, specific genes on the X chromosome can be silenced if they carry mutations, impacting their health. Understanding X chromosome inactivation helps in developing targeted therapies for these male patients too.
What potential does chromosome therapy offer for diseases related to X chromosome inactivation?
Chromosome therapy, particularly in the context of X chromosome inactivation, holds significant potential for treating genetic diseases like Fragile X and Rett Syndromes. By manipulating the inactivation process or unsilencing specific genes, researchers hope to correct genetic errors and restore normal functionality to affected individuals, paving the way for new clinical interventions.
How can understanding X chromosome inactivation lead to breakthroughs in treating Rett Syndrome?
Understanding X chromosome inactivation is crucial for developing potential treatments for Rett Syndrome, which is often linked to mutations on the X chromosome. Jeannie Lee’s research indicates that by unsilencing the inactive X chromosome, it might be possible to restore healthy gene function and mitigate the neurodevelopmental impacts of this disorder.
What are the future directions for research on X chromosome inactivation in relation to genetic diseases?
Future research on X chromosome inactivation aims to further elucidate the mechanisms behind this process and explore novel therapeutic strategies, including chromosome therapy. With promising results from Jeannie Lee’s lab, the focus will shift toward optimizing therapeutic methods and initiating clinical trials to combat X-linked genetic diseases like Fragile X and Rett Syndromes.
| Aspect | Key Points |
|---|---|
| Researcher & Institution | Jeannie T. Lee, Harvard Medical School |
| Main Focus | Understanding X chromosome inactivation and its implications for genetic diseases. |
| X Inactivation Process | Females with two X chromosomes inactivate one, using Xist RNA to alter the chromosome’s environment. |
| Gelatinous Substance | Chromosomes are surrounded by a jelly-like structure essential for proper chromosomal organization and inactivation. |
| Health Implications | Potential therapies for Fragile X Syndrome and Rett Syndrome by ‘unsilencing’ mutated genes on the X chromosome. |
| Progress & Future Plans | Clinical trials for therapies targeting X-linked genetic disorders are anticipated in the coming years. |
| Remaining Questions | Why unaffected genes on the X chromosome remain functional upon unsilencing mutated genes requires further research. |
Summary
X chromosome inactivation is a crucial biological process that affects how female cells manage their extra X chromosome. Recent research by Jeannie T. Lee and her team reveals significant insights into this process and its potential to treat genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. By understanding how Xist RNA alters the properties of the surrounding chromosomal environment, scientists are paving the way toward new therapies that may finally provide relief for individuals affected by these conditions. The ongoing work not only sheds light on the basics of genetics but also promises significant advancements in clinical applications.