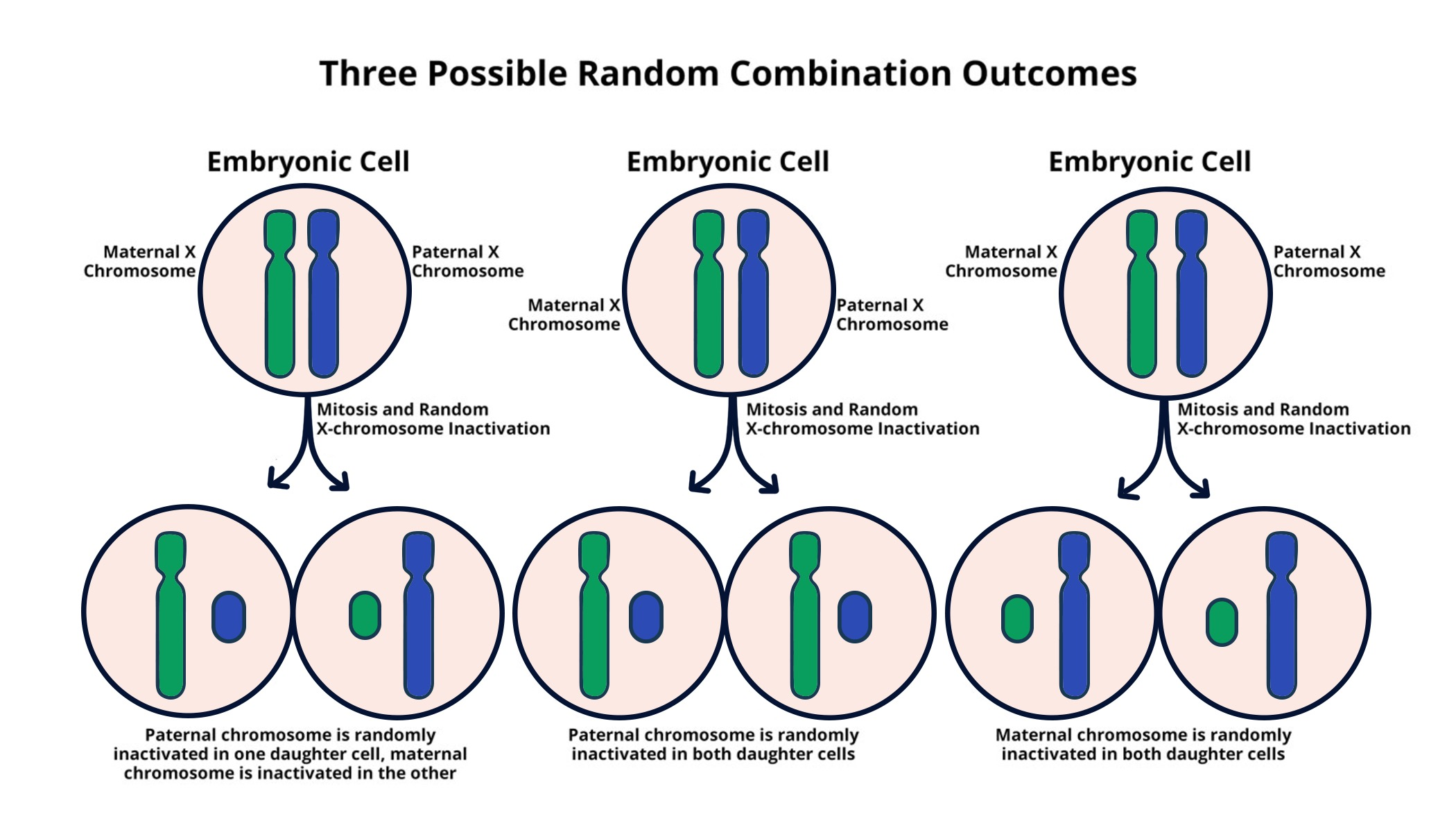
X-chromosome inactivation is a fascinating and complex biological process that plays a crucial role in the genetics of females. In human cells, females possess two X chromosomes, necessitating the silencing of one to prevent an overload of gene dosage. This essential mechanism has been the focus of extensive chromosomal research, with scientists like Jeannie Lee at the forefront of uncovering its intricacies. Recent studies in Lee’s lab suggest that this chromosomal silencing could pave the way for innovative gene therapy treatments for disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. The potential for these breakthroughs not only offers hope for affected individuals but also highlights the intricate relationship between gene regulation and disease management.
The phenomenon of X-chromosome inactivation, sometimes referred to as random X-inactivation, involves a unique genetic strategy that balances the expression of X-linked genes in female mammals. Given that females have two copies of the X chromosome, a regulatory process is needed to ensure that gene expression levels remain comparable to males, who have only one X chromosome. This critical cellular event has sparked significant interest in chromosomal studies, particularly in understanding its implications for genetic diseases. By exploring how genes are silenced on one X chromosome, researchers are uncovering methods to reactivate beneficial genes, providing potential solutions for conditions like Fragile X Syndrome and Rett Syndrome. As researchers like Jeannie Lee continue to investigate the underlying mechanisms, it opens avenues for novel therapeutic strategies in managing these genetic disorders.
Understanding X-Chromosome Inactivation
X-chromosome inactivation (XCI) is a crucial biological process that ensures equal expression of X-linked genes in females, who possess two X chromosomes, compared to males with only one. This phenomenon serves to balance the dosage of genes found on the X chromosome, a necessity given the potential redundancy of possessing two copies. The inactivation occurs early in development and is maintained throughout the organism’s life, making it a significant focus of cellular and genetic research. Jeannie Lee’s groundbreaking work has provided insight into the mechanisms underlying this complex process, particularly how certain molecules, including Xist RNA, play pivotal roles in orchestrating the inactivation of one of the X chromosomes, effectively silencing it.
Researchers are continually exploring the nuances of XCI as it holds broader implications not just for genetics, but also for disorders linked to X chromosome mutations, including Fragile X Syndrome and Rett Syndrome. These disorders exemplify how the malfunctioning of X-linked genes can lead to severe developmental issues, making the understanding of XCI vitally important. By dissecting how XCI operates, scientists hope to unlock new therapeutic avenues to alleviate or potentially cure these genetic disorders.
The Role of Gelatinous Material in X-Chromosome Dynamics
A notable finding in Jeannie Lee’s research highlights the role of a gelatin-like substance that encases chromosomes, which has been likened to Jell-O. This material is essential in creating a space around chromosomes, facilitating the intricate dynamics that occur during X-inactivation. The flexibility of this gelatinous structure allows for the traversal of key proteins and RNA molecules necessary for the silencing of the X chromosome. As Xist RNA engages with this substance, it undergoes a transformation that alters the biophysical properties, making it crucial for effective inactivation of the X chromosome. This understanding reveals how the local environment of chromosomes significantly influences cellular processes.
Moreover, this Jell-O-like material is not solely a passive bystander. It interacts actively with nascent signaling molecules like Xist, playing a collaborative role in the gene expression landscape. Disturbances to this gelatinous coating can result in aberrant gene activity or X-linked disorders, indicating its importance in maintaining genomic stability. As researchers delve deeper into the mechanics of this gelatinous substance, there is potential for significant medical advancements, particularly in developing gene therapies targeted at genetic conditions such as Fragile X Syndrome and Rett Syndrome.
Implications for Gene Therapy in Fragile X and Rett Syndromes
The exploration of X-chromosome inactivation has profound implications for developing gene therapies aimed at conditions like Fragile X Syndrome and Rett Syndrome. Mass General’s Jeannie Lee has opened new avenues by investigating how unsilencing inactivated X chromosomes can restore the function of crucial genes that carry mutations causing these disorders. For individuals with Fragile X syndrome, where a single gene mutation can lead to intellectual disabilities, pursuing treatments that reactivate the silenced gene on the X chromosome has the potential to provide desperately needed relief. With Lee’s lab poised to transition from basic research to clinical applications, we might soon see tangible advancements in therapeutic strategies that could transform patients’ lives.
Furthermore, the therapeutic potential extends beyond female patients since the mechanisms of XCI can still leave male patients vulnerable to mutations on their single X chromosome. By understanding and harnessing the genetic underpinnings of XCI, therapies can be designed that target both sexes effectively. This dual approach encourages innovative solutions to tackle symptoms across a spectrum of conditions tied to X-linked genes, offering hope for broader applications of gene therapy spanning various genetic disorders.
The Future of Chromosomal Research and Genetic Disorders
The journey of chromosomal research has been long and complex, with the study of X-chromosome inactivation as just one compelling chapter. As we advance, ongoing studies are likely to uncover more about how chromosomal interactions occur and how they relate to genetic disorders such as Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s research is paving the way for a new area of understanding regarding how chromosomal abnormalities affect gene expression and how these changes can lead to disease. Looking ahead, this knowledge could revolutionize our approaches to genetic therapies and lead to novel treatment strategies.
In addition to uncovering the mechanisms of inactivation, researchers are employing a variety of techniques, including CRISPR and gene editing, to manipulate gene expression. The integration of such technologies with foundational knowledge about X-chromosome dynamics holds promise for developing innovative therapies that target the root causes of diseases. As we delve deeper into chromosomal research, we not only enhance our understanding of genetics but also edge closer to finding viable solutions for genetic disorders that have long eluded effective treatment.
Challenges in Gene Therapy for X-Linked Disorders
Despite the promising avenues being explored in gene therapy for X-linked disorders, significant challenges remain. One major obstacle is the complexity of how X-chromosome inactivation selectively affects certain genes, leaving some unaffected while silencing others. Jeannie Lee’s investigations suggest that the cellular machinery has limitations on the number of genes it can express simultaneously, creating a balance that must be navigated meticulously in the context of therapy. Therein lies the challenge: designing treatments that can effectively reactivate or correct mutated genes without disrupting the healthy gene expression patterns on the remaining X chromosome.
Moreover, the potential for unintended consequences in gene therapies means rigorous safety studies are essential before these treatments can advance into clinical trials. The Lee lab acknowledges that understanding the exact mechanisms behind the selective silencing of genes is imperative to mitigate risks associated with therapeutic interventions. As researchers continue to unravel these complexities, they remain hopeful that a viable path forward exists, one that might lead to effective treatments with minimal side effects for conditions like Fragile X Syndrome and Rett Syndrome.
Innovative Approaches in Chromosomal Gene Manipulation
As scientists look to harness the power of genetic interventions, innovative approaches are emerging that closely examine chromosomal structures and their functionalities. Techniques such as targeted gene editing and RNA therapy have gained traction in recent years, as researchers aim to create precision treatments for X-linked disorders. Jeannie Lee’s work exemplifies this progressive focus, zeroing in on the cellular components involved in X-chromosome inactivation and how they can be manipulated to achieve therapeutic outcomes.
Recent advances in technology, including synthetic biology and advanced imaging techniques, are enabling researchers to observe and influence the behavior of chromosomal substances in real-time. This ability to visualize how cells interact with different molecular components promises to inform future strategies aimed at correcting genetic defects. The ultimate goal is to create adaptable therapies that can be personalized for individuals suffering from conditions such as Fragile X Syndrome and Rett Syndrome, thereby maximizing the potential for positive health outcomes.
Promoting Awareness of X-LINKED Genetic Disorders
As research continues to uncover the complexities surrounding X-linked genetic disorders, there is a critical need for public awareness and understanding of these conditions. Fragile X Syndrome and Rett Syndrome, being among the most well-studied, highlight the devastating impact that mutations on the X chromosome can have on individuals and their families. Organizations focused on advocacy and support play an essential role in ensuring families have access to the latest research, resources, and information regarding these disorders. Jeannie Lee’s contributions to this field not only elevate scientific discourse but also foster a broader conversation regarding the implications of genetic findings.
Encouraging dialogues about the genetic aspects of these disorders can lead to enhanced support systems for affected individuals. Raising awareness helps dispel misinformation and empowers families, equipping them with insights into potential treatment options or clinical trials that may be available. As the research community, healthcare providers, and advocates work collectively to shed light on X-linked genetic disorders, they create an environment ripe for collaboration, innovation, and ultimately, better health outcomes for those affected by such challenges.
Ethical Considerations in Genetic Research and Therapy
With the rapid advancements in genetic research and therapy, ethical considerations have become increasingly important. As Jeannie Lee and her colleagues explore methods to manipulate X-chromosome inactivation for therapeutic purposes, the implications of such interventions must be carefully examined. Balancing the potential benefits of gene therapy with the risks of unintended consequences is an ongoing challenge that researchers must navigate. Questions of consent, equitable access to therapies, and the long-term impacts of genetic manipulation also demand attention.
The responsible conduct of research and clear communication of findings to the public are paramount in maintaining trust and transparency. Ethical frameworks that guide decision-making in genetic research can help ensure that scientific progress aligns with societal values and that the application of such technologies to treat disorders like Fragile X Syndrome and Rett Syndrome is approached with caution and integrity. Engaging a diverse range of stakeholders in these discussions is essential to foster dialogue and understanding about the complexities of genetic interventions.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important for understanding Fragile X Syndrome?
X-chromosome inactivation is a biological process in female mammals where one of the two X chromosomes is randomly inactivated to ensure dosage compensation between sexes. This mechanism is crucial for understanding Fragile X Syndrome, as mutations on the X chromosome can lead to intellectual disabilities and other symptoms. By studying X-chromosome inactivation, researchers can potentially develop gene therapies to reactivate healthy genes in individuals with Fragile X Syndrome.
How is Xist RNA involved in X-chromosome inactivation and its implications for gene therapy?
Xist RNA plays a pivotal role in X-chromosome inactivation by coating the X chromosome and altering nearby chromosomal material, thus silencing it. This discovery by Jeannie Lee’s lab opens avenues for gene therapy, as it may allow scientists to unsilence mutated X-linked genes, potentially offering treatments for conditions like Fragile X Syndrome and Rett Syndrome.
What recent breakthroughs have been made in chromosomal research related to X-chromosome inactivation?
Recent breakthroughs in chromosomal research include the identification of how Xist RNA interacts with chromosomal material to facilitate X-chromosome inactivation. Jeannie Lee’s lab has demonstrated that this process could potentially be reversed, which is promising for developing therapies for genetic disorders caused by mutations on the X chromosome, such as Fragile X Syndrome.
How could understanding X-chromosome inactivation contribute to treatments for Rett Syndrome?
Understanding X-chromosome inactivation could significantly impact the treatment for Rett Syndrome by providing insights into how to reactivate healthy genes within the inactivated X chromosome. Research by Jeannie Lee suggests that methods to unsilence these genes could lead to innovative therapies that alleviate symptoms in individuals affected by Rett Syndrome.
Why is the study of chromosomal silencing essential for future gene therapy advancements?
The study of chromosomal silencing, specifically regarding X-chromosome inactivation, is essential for future gene therapy advancements because it reveals mechanisms by which mutations on chromosomes can be compensated for. By learning how to manipulate these processes, researchers could develop targeted therapies to reactivate healthy alleles that are otherwise silenced, making gene therapy more effective for conditions like Fragile X Syndrome and Rett Syndrome.
| Key Concept | Details |
|---|---|
| X-Chromosome Inactivation (XCI) | Females have two X chromosomes but only need the gene activity from one, leading to the inactivation of the second copy to prevent gene dosage imbalance. |
| Role of RNA (Xist) | Xist RNA plays a crucial role in marking one of the X chromosomes for inactivation by modifying the chromosomal environment, making it less dense and more flexible. |
| Gelatinous Substance | The ‘Jell-O’ substance surrounding chromosomes is key for proper chromosomal function and separation, preventing entanglement during cell division. |
| Potential Therapies | Research into reversing XCI may lead to treatments for diseases like Fragile X Syndrome and Rett Syndrome, targeting mutated genes while preserving healthy ones. |
| Ongoing Research | The Lee lab continues working on methods to unsilence genes on the X chromosome, aiming for clinical trials in the near future. |
Summary
X-chromosome inactivation (XCI) is a crucial process in female mammals where one of the two X chromosomes is silenced to ensure gene dosage balance between sexes. This mechanism has been further elucidated by recent research led by Jeannie Lee, whose team has demonstrated how a gelatinous substance surrounding chromosomes facilitates this inactivation through the RNA molecule Xist. Understanding XCI opens up potential therapeutic avenues for genetic disorders associated with mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. The ongoing efforts to reverse XCI could lead to significant advancements in treating these conditions, making the research imperative for future medical interventions.